AVISE APS
The Advanced Antiphospholipid Syndrome Diagnostic Test
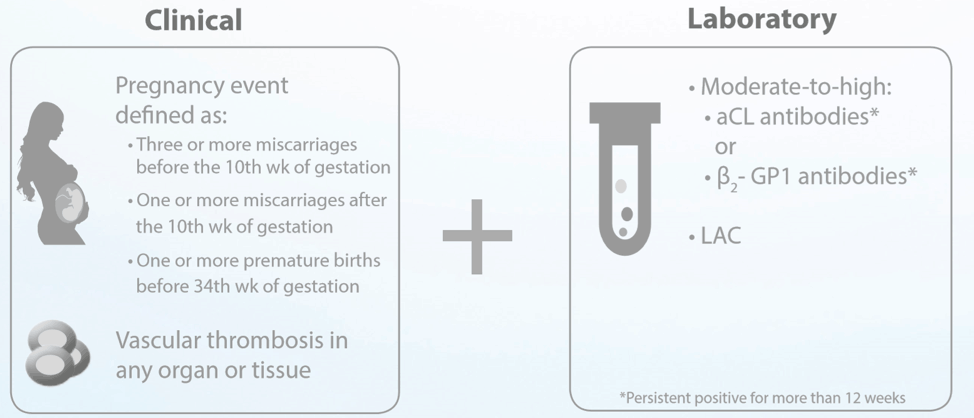
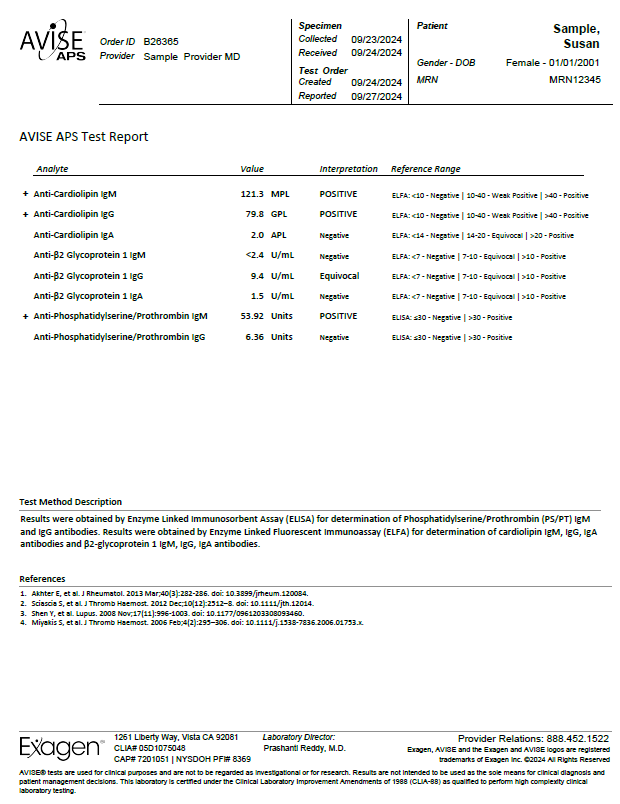
The AVISE APS test is an advanced antiphospholipid syndrome (APS) diagnostic test composed of a combination of biomarkers including, anti-phosphatidylserine/prothrombin (PS/PT) to help assess a patient's risk for APS and thrombosis.
AVISE APS includes the following analytes:
Antiphospholipid syndrome (APS) Classification Criteria:
Key Takeaways from the American College of Rheumatology's 2020 Guidelines on Reproductive Health
Women with rheumatic and musculoskeletal diseases (RMD) have unique concerns associated with reproductive health. The American College of Rheumatology (ACR) has published evidence-based recommendations that can inform reproductive health care in RMD patients, with the goal of minimizing risks and improving maternal and fetal outcomes1.
Importantly, women with RMD should be aware of their antiphospholipid antibody (aPL) status, which can have a substantial impact on how reproductive health is managed. Antiphospholipid syndrome (APS) is the presence of persistent aPL, and it has several obstetrical complications, including recurrent early miscarriages, stillbirths, and late pregnancy complications like preeclampsia and premature delivery2.
Here are our key takeaways from the 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases.
Contraceptives
All women with RMD are strongly recommended to use copper or progestin intrauterine devices (IUDs), including those receiving immunosuppressive therapy. IUDs are safe and highly effective for women with RMD.
Before using any estrogen-containing contraceptive, women with RMD should have an antiphospholipid antibody (aPL) test done. The presence of aPL contraindicates the use of estrogen, which may increase thrombosis risk.
Women with systemic lupus erythematosus (SLE) or aPL are urged to avoid combined estrogen- progestin contraception and the transdermal estrogen-progestin patch; the ACR strongly recommends use of an IUD or progestin-only pill.
Women who are at risk for osteoporosis should avoid intramuscular DMPA injections. Assisted reproductive technology
RMD itself generally doesn’t affect fertility, but some drugs (e.g. cyclophosphamide) might. For women with stable, uncomplicated RMD who are aPL negative, the ACR strongly recommends ART. However, women with active RMD should defer ART until the disease is stable/quiescent.
The ACR has made additional recommendations for women with positive aPL who undergo ART:
- Prophylactic anticoagulation therapy is conditionally recommended for asymptomatic women with no prior thromboses or OB APS, or who have a history of OB APS.
- Therapeutic anticoagulation therapy is strongly recommended in women with prior thromboses.
Fertility Preservation
Embryo and oocyte cryopreservation are strongly recommended for women with unstable RMD, with the following conditional recommendations:
- Gonadotropin-releasing hormone agonist therapy should be used during cyclophosphamide (CYC) treatment.
- CYC treatment should be discontinued before oocyte cryopreservation.
Men with RMD should not use gonadotropin-releasing hormone agonist therapy, and sperm cryopreservation should occur before CYC treatment.
Women with RMD who do not have SLE or aPL can safely use hormone replacement therapy (HRT). The ACR conditionally recommends HRT for women with SLE and negative aPL.
Women with positive aPL have additional recommendations:
- Women who have had prior thrombosis or OB APS and who aren’t receiving
- anticoagulation treatment are strongly recommended to not undergo HRT.
- The ACR conditionally recommends that women with aPL generally avoid HRT.
- Women with current negative titers may be candidates for HRT, but some conflicting data exist.
Pregnancy
GPS dictate that all women receive counseling about pregnancy planning and management, including disease stability, compatible medications, and the importance of collaborations between rheumatology and obstetrics-gynecology/maternal-fetal medicine.
Before pregnancy, the ACR strongly recommends that medications be switched to pregnancy-
compatible alternatives to ensure they’re effective and well tolerated.
Further recommendations for women with SLE:
- An early test for any of the three types of aPL (aCL, anti-β2GPI, LAC) is strongly
- recommended for women with SLE and SLE-like diseases.
- A conditional recommendation can be made for the use of hydroxychloroquine during pregnancy, if there are no contraindications.
- Further recommendations for women with positive aPL:
- A recommendation was made for the use of prophylactic aspirin (81 or 100 mg daily) throughout pregnancy as preeclampsia prophylaxis.
- Women who meet criteria for OB APS are strongly recommended to combine low-dose aspirin with a prophylactic-dose anticoagulant.
Anticoagulant therapy should continue for 6-12 weeks post-partum.
- Women with thrombotic APS are strongly recommended to combine low-dose aspirin with a therapeutic-dose anticoagulant.
- Evidence does not support the use of prednisone in combination with anticoagulants and aspirin.
In addition, the ACR strongly recommended that women with SLE, SLE-like diseases, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis be tested early for anti-Ro/SSA and anti- La/SSB.
Medications
The ACR made several recommendations about use of medications.
1. Men:
- Strongly recommend continuing hydroxychloroquine, azathioprine, colchicine, and tumor necrosis factor inhibitors (all).
- Conditionally recommend continuing anakinra, cyclooxygenase-2 inhibitors, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil, mycophenolic acid, nonsteroidal anti-inflammatory drugs, rituximab, sulfasalazine (semen analysis if delayed conception), and tacrolimus.
- Strongly recommend discontinuing CYC.
- Conditionally recommend discontinuing thalidomide.
2. Women:
- Strongly recommend continuing hydroxychloroquine, sulfasalazine, azathioprine, colchicine, and certolizumab.
- Conditionally recommend continuing cyclosporine, infliximab, etanercept, adalimumab, golimumab, low-dose prednisone, and tacrolimus.
- Strongly recommend discontinuing methotrexate, mycophenolate mofetil, thalidomide, leflunomide, and CYC prior to conception.
- Conditionally recommend discontinuing rituximab, belimumab, anakinra, abatacept, tocilizumab, secukinumab, and ustekinumab once pregnancy is confirmed.
- It is strongly recommended that NSAIDs be avoided in the 3rd trimester.
The ACR encourages breastfeeding for women with RMD, with the acknowledgement that benefits of disease control need to be balanced with the risk of exposing an infant to medications through breast milk. Hydroxychloroquine, colchicine, sulfasalazine, rituximab, and all TNF inhibitors are compatible with breastfeeding. Drugs that are not compatible with breastfeeding include CYC, leflunomide, MMF, and thalidomide.
Conclusion
Although they may face some unique challenges, women with RMD have a great chance at conceiving and delivering a healthy baby when appropriate precautions are taken. The ACR guidelines repeatedly stressed the importance of having a multidisciplinary health care team that includes specialists from rheumatology, obstetrics-gynecology, and maternal-fetal medicine.
The ACR also emphasized the need for aPL testing among women of reproductive age; a positive aPL test has implications for more than pregnancy. aPL-positive women who use estrogen-based birth control increase their risk for thrombosis. The AVISE APS test is an advanced APS diagnostic test that can help inform reproductive health care strategies for women, whether their goal is contraception or delivering a healthy baby.
References
References:
- Antiphosphatidylserine/Prothrombin (PS/PT)Akhter et al. Utility of Antiphosphatidylserine/Prothrombin and IgA Antiphospholipid Assays in Systemic Lupus Erythematosus. Journal of Rheumatism. 2013.
-
Sammaritano, L.R., et al., 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol, 2020. 72(4): p. 529-556.
-
Schreiber, K., et al., Antiphospholipid syndrome. Nat Rev Dis Primers, 2018. 4: p. 17103.
SA1214 (7/20)