AVISE HCQ
Hydroxychloroquine (HCQ)
Test Measuring Drug Levels
in Whole Blood
AVISE HCQ is an advanced drug monitoring test that provides accurate measurement of hydroxychloroquine (HCQ) levels in whole blood to help physicians assess their patients' exposure to hydroxychloroquine therapy.
Clinical Utility
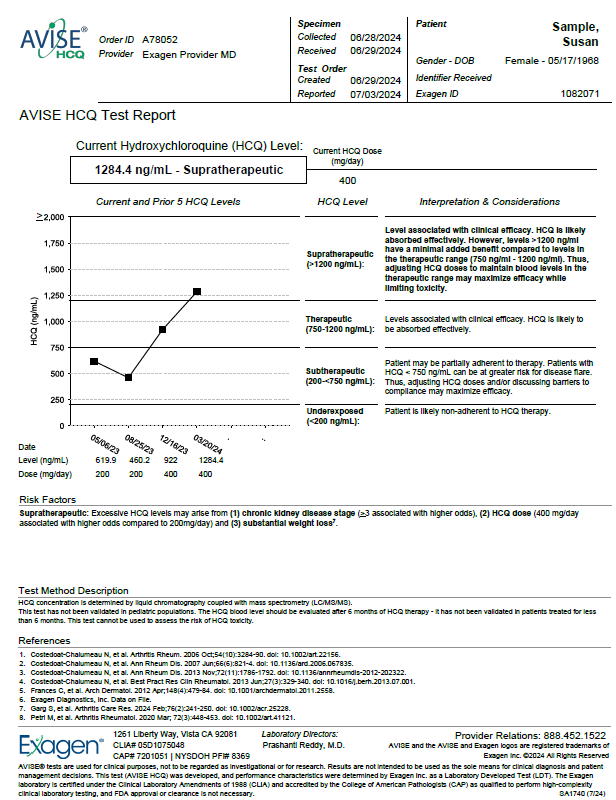
AVISE HCQ Test Report
The AVISE HCQ test is intended for use in patients on hydroxychloroquine (PLAQUENIL) therapy, after steady state (approx. 90-120 days), to help assess the following:
Patient risk for disease flares
Therapeutic decision making
Hydroxychloroquine
Hydroxychloroquine (HCQ) has been successfully used to treat malaria for decades, but it’s also a common treatment for autoimmune disorders like rheumatoid arthritis. When used appropriately, HCQ is very safe, and it’s included on the World Health Organization’s List of Essential Medications. However, HCQ use is not without some risk, so it’s important that people who take HCQ be aware of the dangers of toxicity and how to spot it. People who take HCQ long-term should have intermittent blood tests to make sure their HCQ level is within the appropriate therapeutic range.
HCQ has been in the news recently as a potential treatment for COVID-19, but whether it is truly effective remains to be determined. At this time, the U.S. Food and Drug Administration (FDA) is recommending that HCQ be used as a COVID-19 treatment only in a hospital setting1. This is not only to protect COVID-19 patients from potential heart rhythm problems and other unforeseen complications, but also because people who have relied on HCQ to help them manage chronic disorders are struggling to get it in the current high-demand environment.
What is hydroxychloroquine?
HCQ is in a family of drugs called antimalarials, and it is an effective way to prevent and treat acute attacks of malaria2. It also belongs to a class of drugs called disease modifying anti-rheumatic drugs, which are anti-inflammatory medications that can reduce symptoms associated with autoimmune conditions like rheumatoid arthritis and lupus.
Why is it used?
HCQ has been approved by the FDA to treat the following conditions:
• Malaria
• Rheumatoid arthritis (RA)
• Systemic lupus erythematosus (SLE)
RA and SLE are autoimmune disorders, which occur when the immune system mistakenly attacks normal, healthy cells and tissue. While the mechanisms still aren’t completely understood, HCQ can calm an overactive immune system. It is thought to work by interrupting some of the signals that activate the immune system and reducing the amount of pro-inflammatory chemicals that are released by immune cells3. For people with autoimmune disorders, HCQ can be an effective way to manage their symptoms.
Dangers of toxicity
HCQ is generally very safe when used as directed, but it’s important for people taking HCQ to be aware of the signs of HCQ toxicity and the dangers of overdose.
HCQ has a long half-life, about 50 days3. This means it takes your body 50 or so days to metabolize half of the HCQ in your system, and another 50 days to metabolize half of the remaining HCQ. People who take large doses or who use HCQ for a long time are at particular risk for toxicity, since HCQ accumulates in several organs, including the eye, the skin, and the liver.
Since HCQ accumulates over time, an overdose is more likely to be associated with long-term use than an acute episode. Mild overdoses are characterized by gastrointestinal upset, headache, and a rash.
More serious overdose symptoms include visual and/or hearing disturbances, irregular heartbeat, and muscle weakness.
One of the most serious concerns of long-term HCQ use is the development of retinopathy. HCQ preferentially accumulates in melanin-containing organs like the eye, where it can cause irreversible damage to the retina and cause partial loss of vision and visual disturbances like halos or glare.
Studies have shown that using HCQ for more than 5 years significantly increases the risk for development of HCQ-induced toxic retinopathy4. Unfortunately, retinopathy may progress even if HCQ is discontinued, both because HCQ can take up to a year to be fully eliminated from the body and because retinal degradation may continue.
HCQ blood testing to prevent retinopathy
People who rely on HCQ for the long-term treatment of autoimmune conditions should be tested intermittently to keep track of HCQ accumulation. Until recently, plasma tests were typically used to determine HCQ levels, but since HCQ binds to red blood cells, plasma tests return artificially low results. Whole blood tests are a more accurate way to determine HCQ levels.
Studies have shown that someone who takes 400 mg HCQ per day (a standard dose for autoimmune disorders) will accumulate about 1 kilogram of HCQ in their organs after 7 years, which is linked to an increased risk for retinopathy5. Regular blood testing gives doctors a way to identify the lowest effective dose and tailor it as needed to ensure that patients get the best outcomes with the lowest risks.
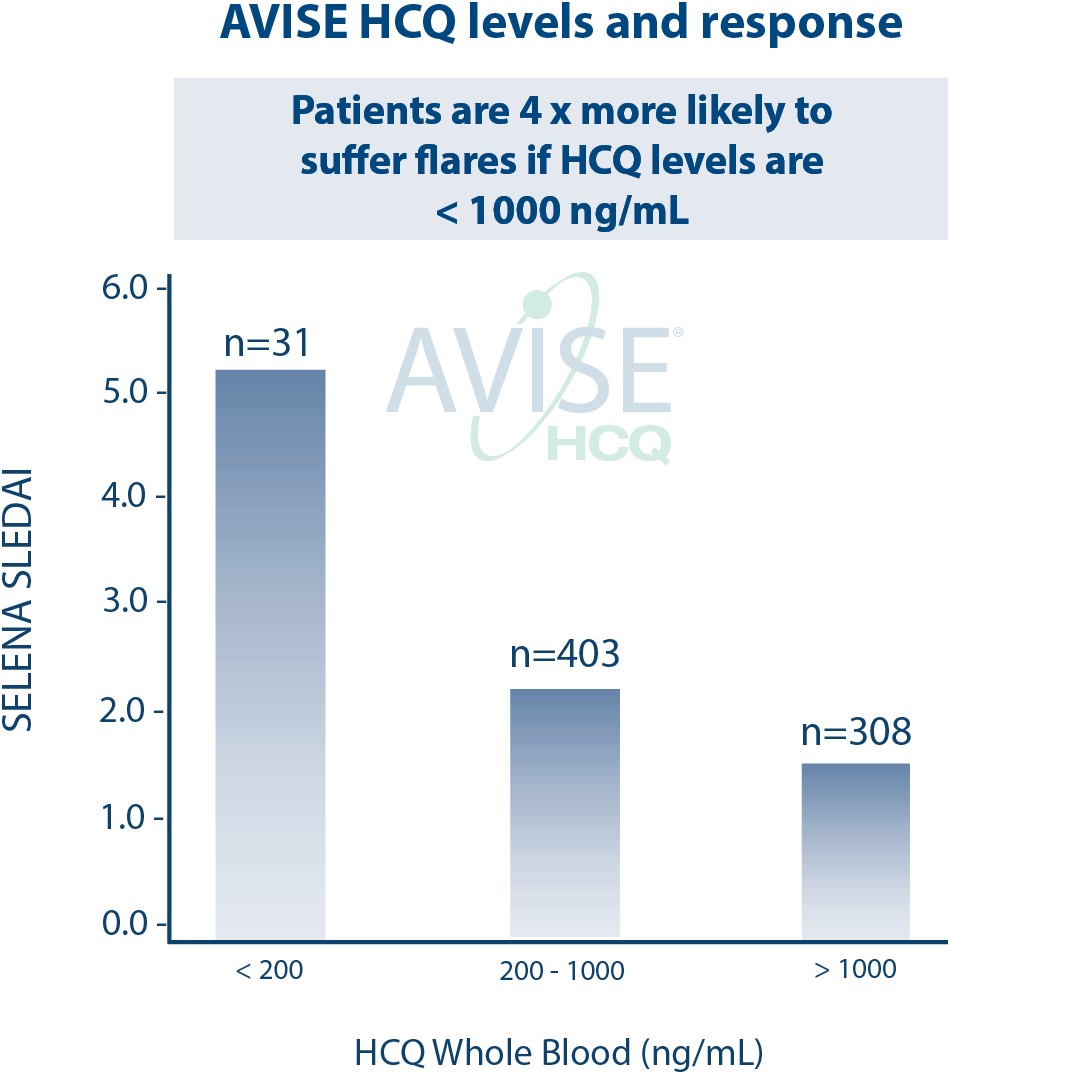
In addition, whole blood HCQ tests have been shown to predict retinopathy6. For people who have been on HCQ for several years, getting an HCQ blood test could identify their risk for developing retinopathy and help inform treatment plans to maximize HCQ efficacy and minimize risks.
AVISE HCQ is an advanced drug monitoring test that provides an accurate measurement of HCQ levels in whole blood. This allows for precise dosing to make sure a patient’s symptoms are being managed, without putting them at risk for complications that are caused by too little or too much HCQ.
References:
- Costedoat-Chalumeau N, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum. 2006 Oct;54(10):3284-90.
- Costedoat-Chalumeau N, et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann Rheum Dis. 2007 Jun;66(6):821-4.
- Costedoat-Chalumeau N, et al. (2013a) Hydroxychloroquine in Systemic Lupus Erythematosus: Results of a French Multicentre Controlled Trial (PLUS Study). Ann Rheum Dis 72:1786-1792.
- Costedoat-Chalumeau N, et al. (2013b) Adherence to Treatment in Systemic Lupus Erythematosus Patients. Best Pract Res Clin Rheumatol 27:329-340.
- Frances C, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol. 2012 Apr;148(4):479-84.
- Exagen Diagnostics, Inc. Date on File.
- MedlinePlus. Hydroxychloroquine. 2020 [cited 2020 May 11].
- Schrezenmeier, E. and T. Dörner, Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nature Reviews Rheumatology, 2020. 16(3): p. 155-166.
- Melles, R.B. and M.F. Marmor, The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol, 2014. 132(12): p. 1453-60.
- Stokkermans, T. and G. Trichonas, Chloroquine And Hydroxychloroquine Toxicity. 2019, Treasure Island, FL: StatPearls Publishing LLC.
- Petri, M., et al., Hydroxychloroquine Blood Levels Predict Hydroxychloroquine Retinopathy. Arthritis Rheumatol, 2020. 72(3): p. 448-453.
SA1215 (7/20)