This is a website for healthcare professionals.
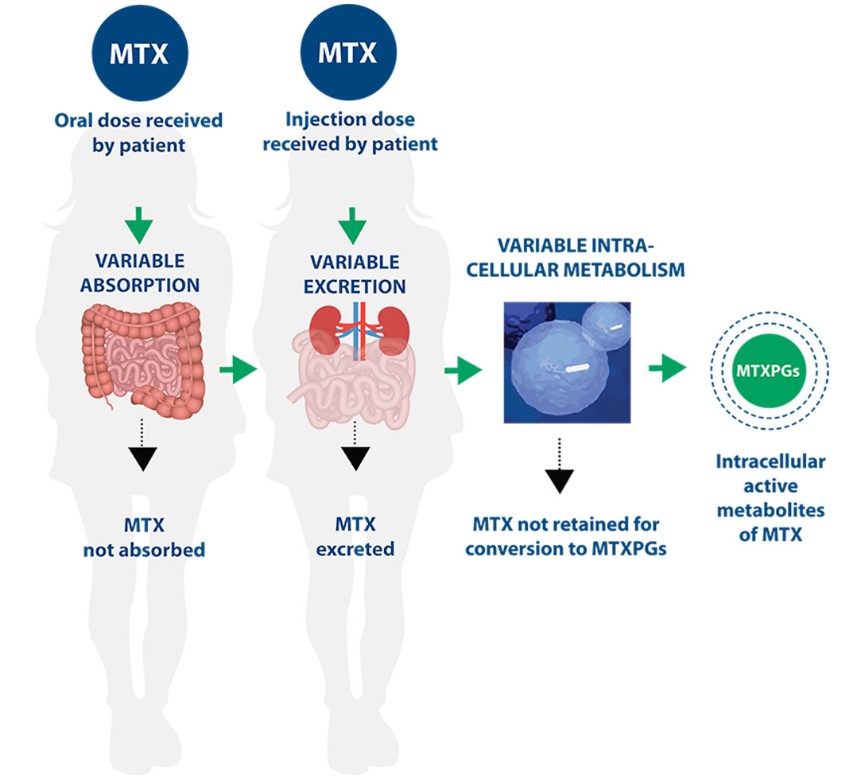
AVISE MTX helps assess variability in methotrexate pharmacokinetics.
Clinical Utility
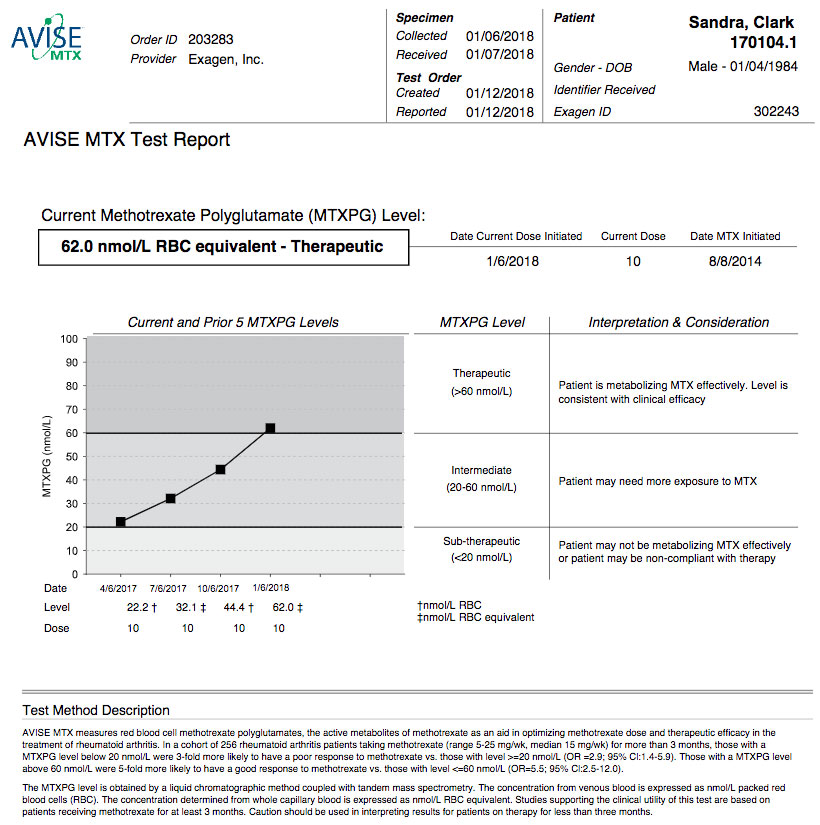
AVISE MTX Test Report
The AVISE MTX test is intended for use in patients on methotrexate treatment, after steady state (approx. 90 days), to help with therapeutic decision making including:
- Drug delivery route, i.e. oral vs. subcutaneous MTX therapy
- Treatment changes
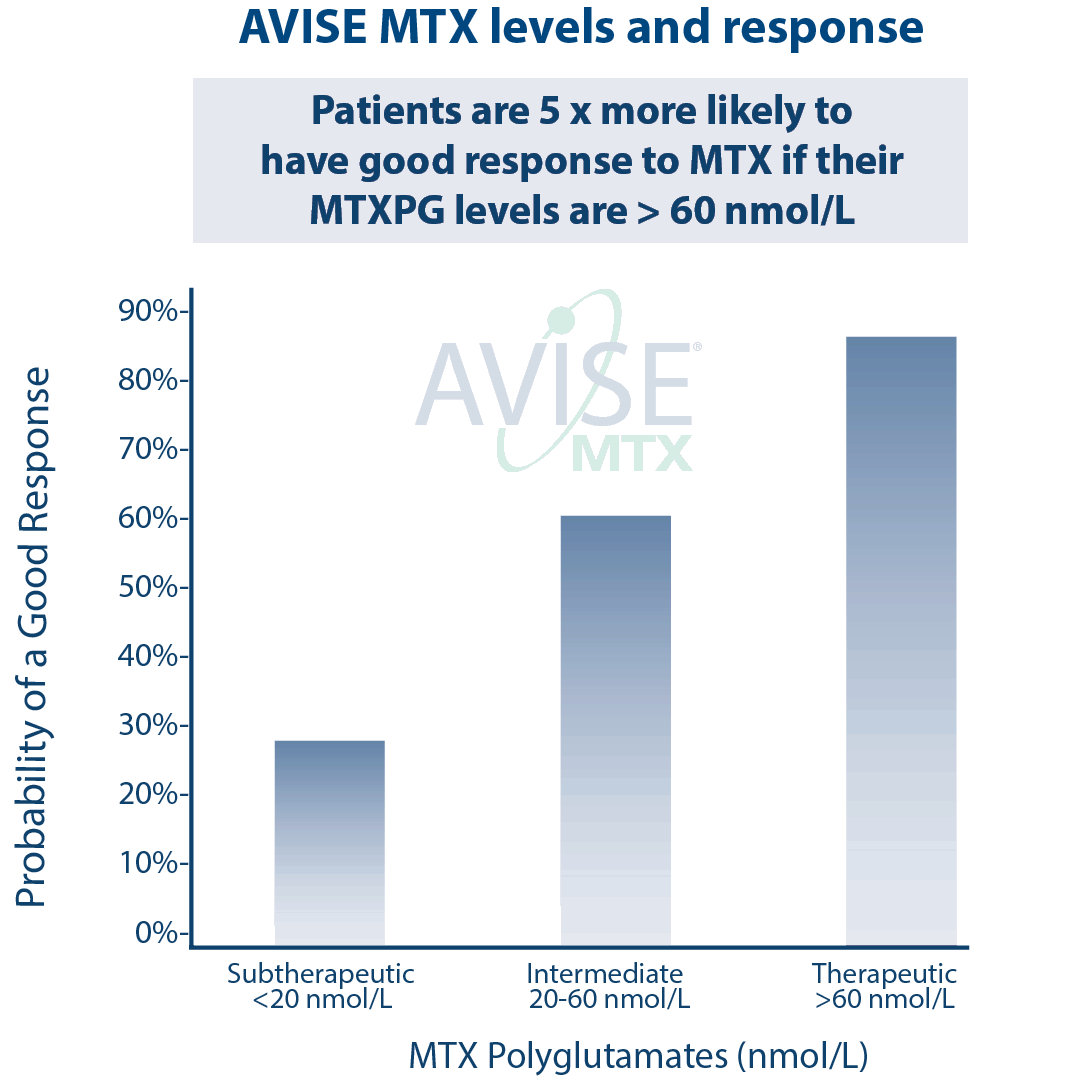
2. Dervieux T, et al. Ann Rheum Dis 2005.
References:
- Dervieux T, Furst D, et al. Polyglutamation of Methotrexate With Common Polymorphisms in Reduced Folate Carrier, Aminoimidazole Carboxamide Ribonucleotide Transformylase, and Thymidylate Synthase Are Associated With Methotrexate Effects in Rheumatoid Arthritis, Arthritis Rheum. 2004; 50(9):2766-2774.
- Dervieux T, Furst D, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentered cross sectional observational study, Ann Rheum Dis 2005;64(8):1180-1185.
- Dervieux T, Greenstein N, et al. Pharmacogenomic and Metabolic Biomarkers in the Folate Pathway and Their Association With Methotrexate Effects During Dosage Escalation in Rheumatoid Arthritis, Arthritis Rheum. 2006;54(10):3095-3103.