AVISE T Cell Biomarkers
Although highly specific, conventional systemic lupus erythematosus (SLE) biomarkers often fall short. To address this, Exagen has identified three novel T Cell biomarkers. As part of AVISE CTD, these biomarkers aim to provide clinicians with a more comprehensive offering to help expedite their SLE patients' diagnostic journey.
For patients with SLE, treating the disease early and appropriately is essential for the best chance of remission and comfort management. SLE is a complex, multisystem autoimmune disease, so diagnosis can be challenging, particularly in early cases. Thus, the diagnosis of SLE is based on a combination of clinical symptoms, and laboratory tests.
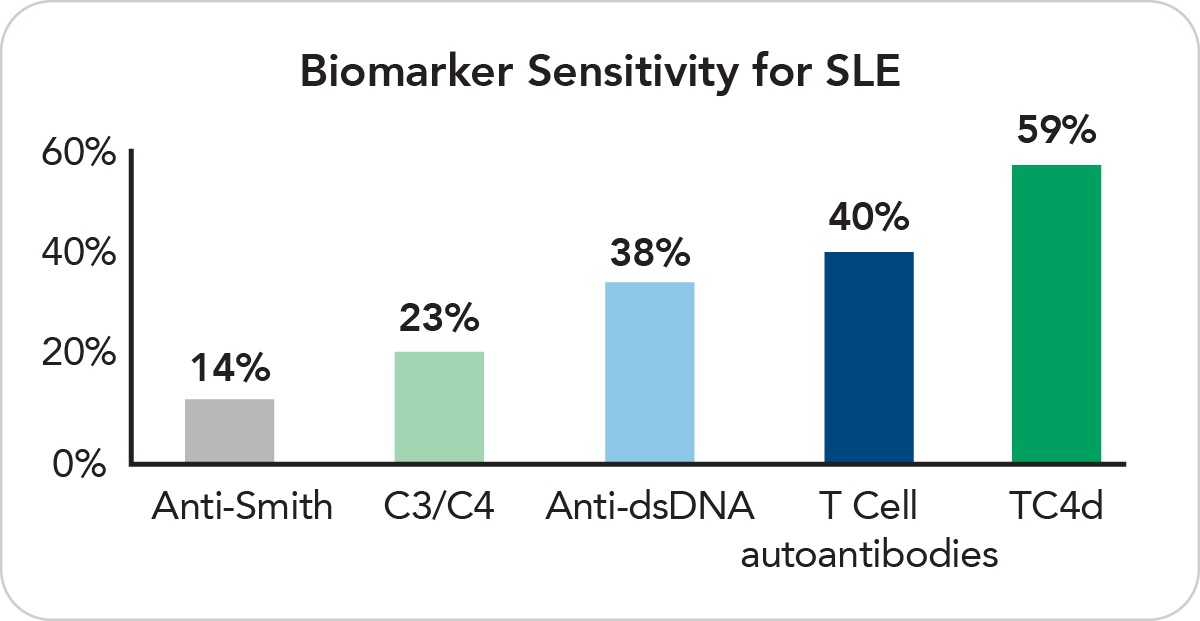
Traditional biomarkers like anti-dsDNA, anti-Smith, and serum complement C3 and C4 often lack the sensitivity required to detect a significant number of early SLE cases. Exagen has addressed this issue by enhancing the comprehensive diagnostic offering, AVISE CTD by adding three new T Cell biomarkers (TC4d, TIgG and TIgM).
Breakthrough T Cell biomarkers provide unique and unmatched diagnostic accuracy for SLE
Incorporating T Cell biomarkers into AVISE CTD enhances the ability to substantiate a clinical suspicion of SLE in situations where conventional labs are inconclusive. These biomarkers add significant value to an already robust diagnostic offering.
Enhanced Sensitivity
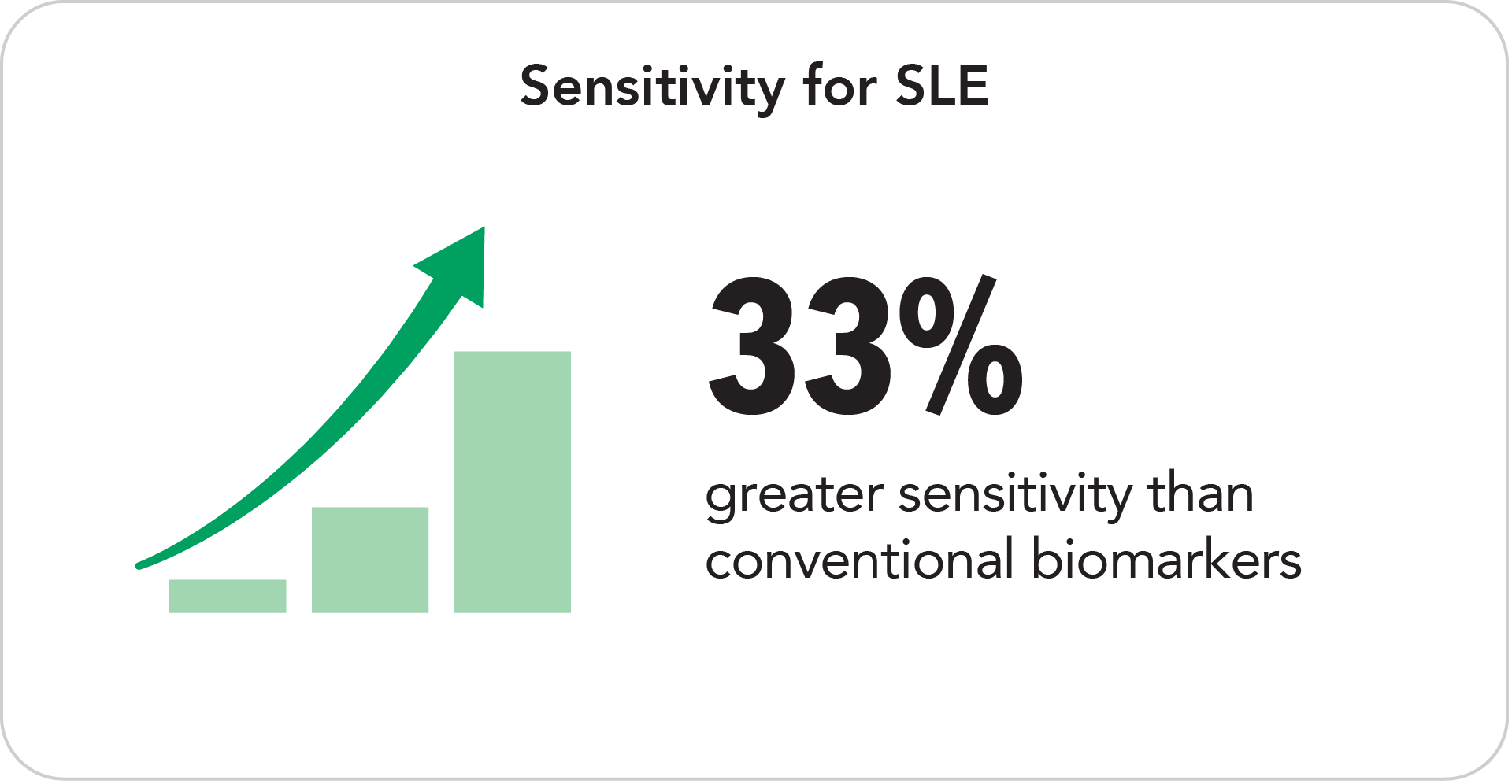
T Cell biomarkers offer greater sensitivity as compared to conventional biomarkers. TC4d is positive in 59% of SLE patients and T Cell autoantibodies are positive in 40%.
Diagnostic Confidence
The addition of T Cell biomarkers to AVISE CTD provides enhanced diagnostic performance as compared to conventional test methods alone.
Distinct Value
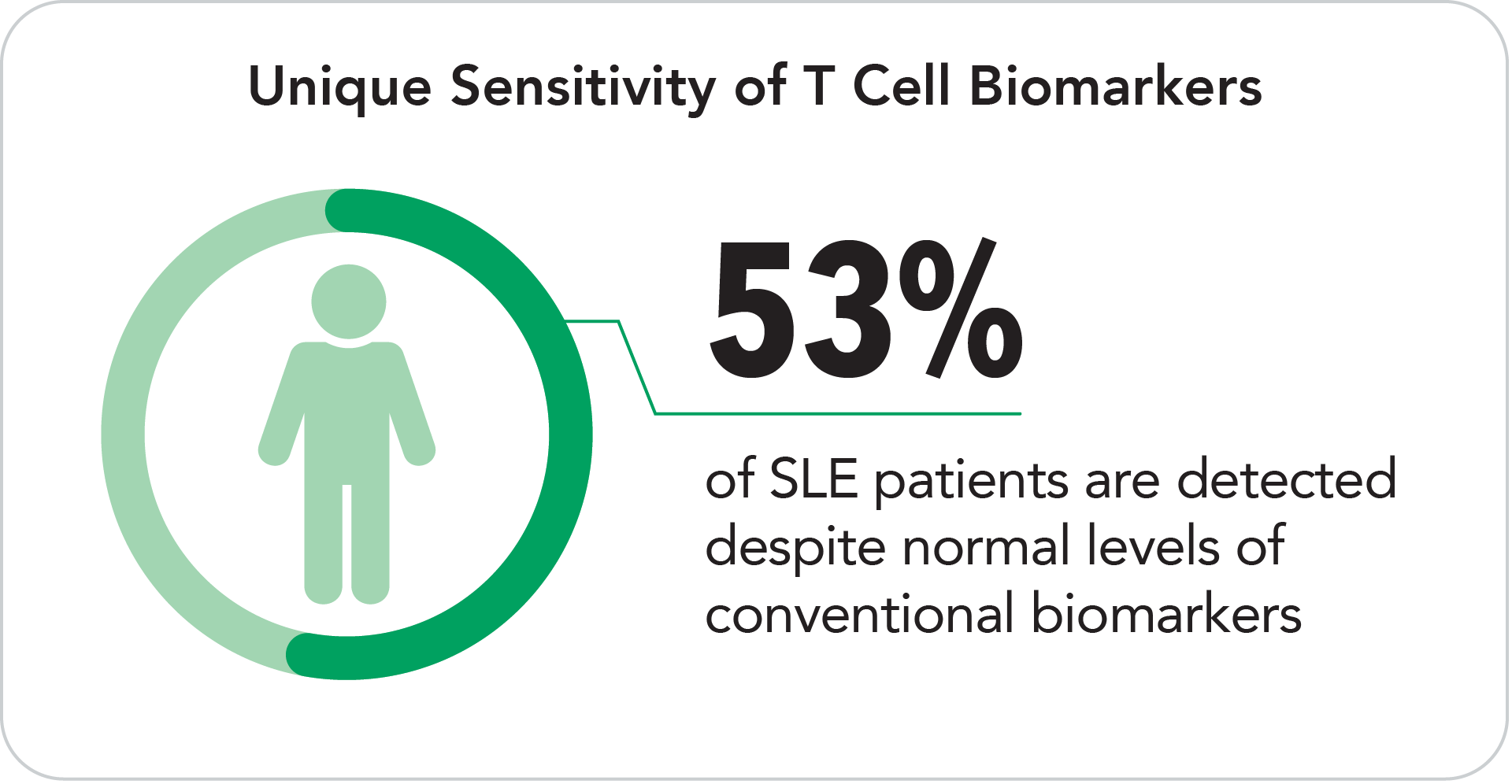
T Cell biomarkers bring additional value by providing independent positivity for SLE patients who test negative for conventional biomarkers.
Why T Cells?
Alterations to normal T Cell function have been implicated in the pathogenesis of SLE. In SLE, T Cells display heightened activation, produce increased levels of pro-inflammatory cytokines, and have reduced regulatory function. This leads to systemic inflammation and tissue damage, which is characteristic of SLE. By studying their role in immune dysregulation, these biomarkers can enhance early detection and monitoring of disease activity in SLE patients.
What is TC4d?
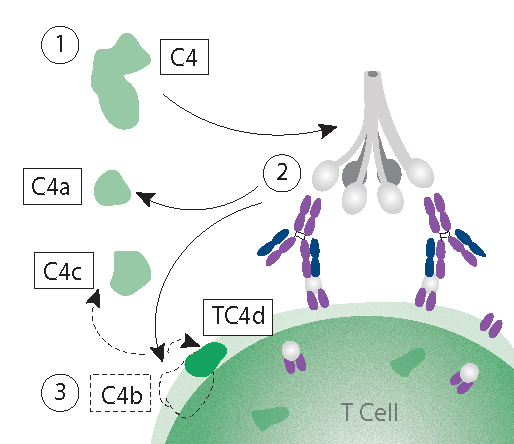
The T Cell-bound complement activation product (TC4d) refers to a specific fragment of the complement component C4, known as C4d. This component is generated during complement activation, which is a key aspect of SLE pathophysiology. The C4 protein is enzymatically degraded upon classical complement activation resulting in the C4d protein. C4d covalently binds to cell surfaces, resulting in a biological signature of SLE.
What are T Cell Autoantibodies?
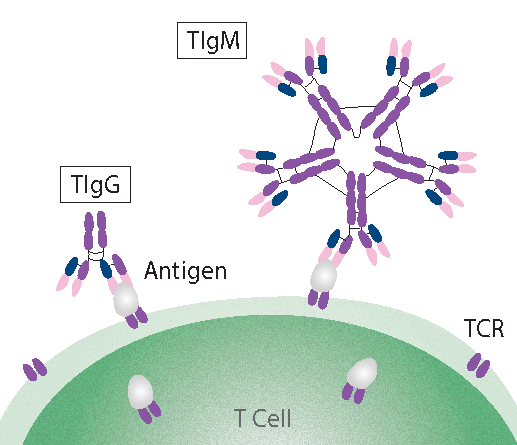
TIgG and TIgM are autoantibodies that bind to antigens on the surface of T Cells, which triggers sublytic complement activation.
Other Resources
Publications
Multi-centered clinical validation demonstrating superior precision in lupus diagnosis: T Cell autoantibodies and TC4d outperform conventional lupus erythematosus biomarkers.
Kyttaris V et al., Front. Immunol. 2025
Novel T Cell biomarkers improved sensitivity for SLE, enabling the identification of additional lupus patients when conventional biomarkers were normal or negative.
Webinars & Videos

ON-DEMAND WEBINAR Innovative Clinical Biomarkers for SLE and RA: Advancing Autoimmune Diagnostics
In this webinar, you will gain insights into emergent biomarkers for systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). The speakers will share how novel T Cell biomarkers can offer more confident diagnoses of SLE, particularly when conventional biomarkers are insufficient.
ON-DEMAND WEBINAR Rethinking Lupus: The Emerging Role of T Cells in SLE Pathophysiology and Diagnostics
This webinar bridges historical insights with modern innovations, providing a comprehensive perspective on SLE pathogenesis and diagnostic advancements.
References:
1. Concoff A, Kyttaris V, Sandhu V, O'Malley T, Casaburi G, Kumar S, Liu C, Manzi S, Ahearn J. Enhancing Systemic Lupus Erythematosus Diagnosis: Comparative Performance Characteristics of TC4d and Anti-T-Cell Antibodies with Conventional Biomarkers [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9).
2. Huang JJ, Mao TJ, Zhang ZY, Feng G. Systemic evaluation of lymphocyte-bound C4d and immunoglobulins for diagnosis and activity monitoring of systemic lupus erythematosus. Clin Biochem. 2023;118:110600. doi:10.1016/j.clinbiochem.2023.110600
3. Liu CC, Manzi S, Ahearn JM. Anti-lymphocyte autoantibodies generate T cell-C4d signatures in systemic lupus erythematosus. Transl Res. 2014;164(6):496-507. doi:10.1016/j.trsl.2014.07.007
4. Kyttaris V, Concoff A, Warsi T, Taghavi S, Kumar S, Park S, Patalinghug A, Schleif C, Partain B, Ahearn J, Wilson N, Liu C, Manzi S, O'Malley T. Multi-centered Clinical Validation of T Cell-bound C4d (TC4d) and T Cell Autoantibodies (TIgG and TIgM): Sensitive and Specific Biomarkers of SLE with Enhanced Accuracy Compared to Conventional SLE Tests [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). Accessed October 4, 2024.
5. Data on File.

