This is a website for healthcare professionals.
AVISE Lupus
Powered by CB-CAPs Technology
AVISE® Lupus is a 10-marker diagnostic test designed to aid healthcare providers in a timely diagnosis of patients with suspected Systemic Lupus Erythematosus (SLE).
AVISE® Lupus is the only lupus test that incorporates Cell-Bound Complement Activation Products (CB-CAPs) assays which have been shown to have higher sensitivity for diagnosing SLE than C3/C4 levels.
This proprietary test also allows for the efficient measurement of essential markers simultaneously and has the opportunity to reduce inconsistencies in diagnostic workups and standardize test results.
AVISE Lupus has the potential to improve the diagnostic landscape for SLE by:
Sample Test Report
AVISE Lupus, consisting of proprietary CB-CAPs and a unique two-tier algorithm, has been shown to improve physician diagnostic confidence, particularly in hard-to-diagnose patients.
How does the AVISE Lupus algorithm work?
AVISE Lupus is a multi-marker algorithmic assay that delivers an index score indicative of SLE likelihood. Our proprietary two-tier algorithm integrates CB-CAPs together with autoantibody levels to provide a balanced diagnostic result with 80% sensitivity and 86% specificity for the diagnosis of SLE.
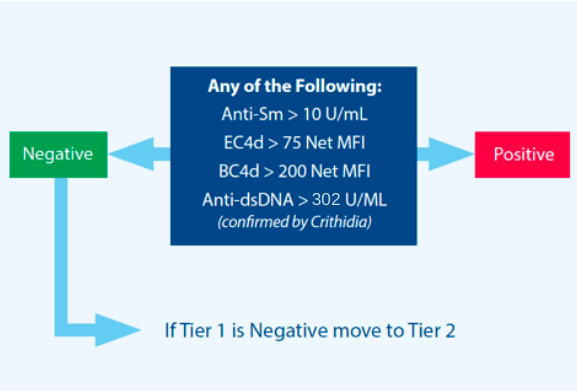
- anti-dsDNA
- anti-Smith
- EC4d or BC4d levels (meeting strong positive criteria)
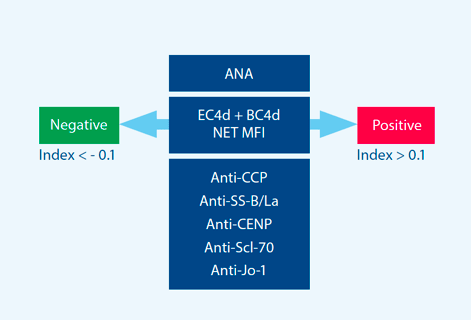
Tier-two provides an index score and assessment aggregated based upon three components: ANA, CB-
CAPs, and autoantibody specificity.
Tier-two includes the specificity component to increase performance in differentiating SLE from
related autoimmune diseases. Key components of the assay include:
- ANA
- EC4d
- BC4d
- Autoantibody specificity components (anti-CCP, anti-SS-B/La, CENP, Jo-1, and Scl-70)
CB-CAPs Provide Increased Accuracy
CB-CAPs, Cell-Bound Complement Activation Products, are complement split products bound to blood cells and include C4d bound to B cells (BC4d), erythrocytes (EC4d), and platelets (PC4d).
AVISE Lupus is the only validated SLE test that incorporates CB-CAPs assays which have been shown to have higher sensitivity for diagnosing SLE than C3/C4, anti-dsDNA, and anti-Smith.
AVISE Lupus provides physicians with greater confidence in diagnostic and treatment decisions than standard diagnostic lab tests. The AVISE Lupus test prompts early initiation of appropriate medication, thereby mitigating the potential for irreversible organ damage and associated decrease in patient quality of life.
References:
- Cell-bound complement activation products in SLE. Ramsey-Goldman R, et al. Lupus Science and Medicine. 2017.
- Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Putterman C. et al. Lupus Science and Medicine. 2014.
- Systemic lupus erythematosus and primary fibromyalgia can be distinguished by testing for cell-bound complement activation products. Wallace D. et al. Lupus Science & Medicine. 2016.
- The AVISE Lupus Test and Cell-bound Complement Activation Products Aid the Diagnosis of Systemic Lupus Erythematosus. Mossell J. et al. The Open Rheumatology Journal. 2016.


