Rheumatoid Arthritis
Rheumatoid Arthritis (RA) is a chronic, systemic autoimmune disorder characterized by persistent synovial inflammation, leading to progressive joint damage, commonly assessed through serologic testing for conventional autoantibodies such as anti-cyclic citrullinated protein (CCP) and Rheumatoid Factor (RF). Up to 30% of patients with RA are seronegative for the classical biomarkers, anti-cyclic citrullinated protein (CCP) and rheumatoid factor (RF). The diagnostic uncertainty in seronegative and early RA often leads to delayed treatment, which increases the risk of joint damage, functional decline, and worsened disease outcomes.
The enhanced RA profile as part of AVISE CTD incorporates anti-CCP and RF biomarkers along with new autoantibody biomarkers that are highly specific to RA and can substantiate an RA diagnosis even when RF and anti-CCP are negative.
The diagnostic uncertainty of seronegative RA, often leads to delayed treatment, increasing the risk of joint damage, functional decline, and worsened disease outcomes. Exagen has addressed this challenge by launching AVISE CTD's enhanced RA profile, which incorporates five new autoantibodies that are highly specific for RA, and can substantiate an RA diagnosis in the absence of anti-cyclic citrullinated peptide (anti-CCP) and rheumatoid factor (RF). Anti-RA33 autoantibodies (IgG, IgM and IgA) target the RA33 protein complex, which plays an important role in gene expression and regulation. Anti-PAD4 (IgA and IgG) autoantibodies target one of the peptidylarginine deiminases (PADs), which are involved in protein citrullination and RA pathogenesis. Both anti-RA33 and anti-PAD4 autoantibodies are found in both seropositive and seronegative RA patients, providing a more complete assessment of individuals with this disease.
Anti-PAD4 autoantibodies offer a novel solution for identifying seronegative RA, enabling earlier diagnosis, clearer differentiation, and prognostic insight to support more confident clinical decisions.
Peptidylarginine deiminases (PADs) play a crucial role in the production of citrullinated proteins, which are the hallmark autoantigens of RA. Anti-PAD4 autoantibodies target one of these enzymes involved in the citrullination process.
Unlike traditional markers, anti-PAD4 can be present in patients who are seronegative for RF and anti-CCP, helping clinicians identify RA earlier in the disease course. By providing additional diagnostic and prognostic insight, anti-PAD4 supports earlier intervention and greater confidence when differentiating RA from other inflammatory arthritides, especially in clinically ambiguous cases.
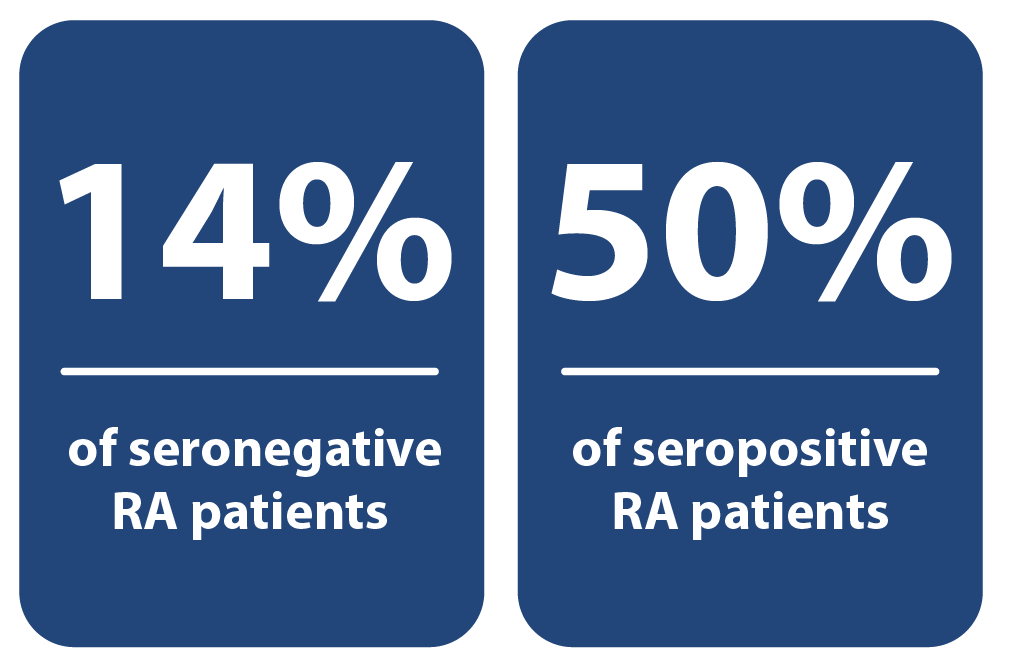
Sensitive
Anti-PAD4 is found in 14% of seronegative and 50% of seropositive RA patients.1
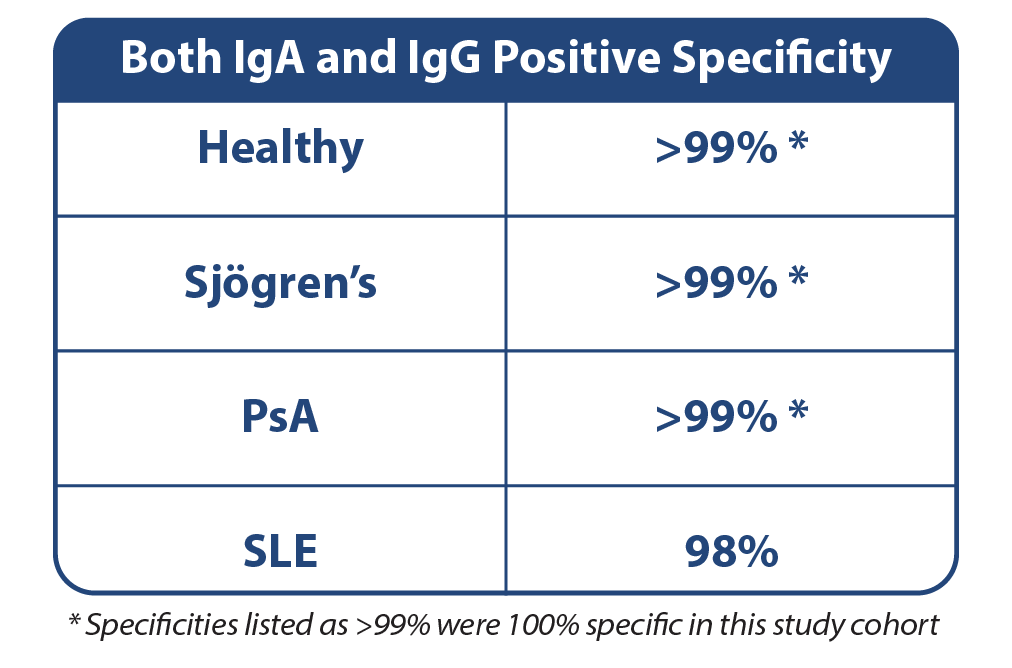
Specific
Coexisting anti-PAD4 IgA and IgG autoantibodies correlate with higher diagnostic specificity for RA. 1
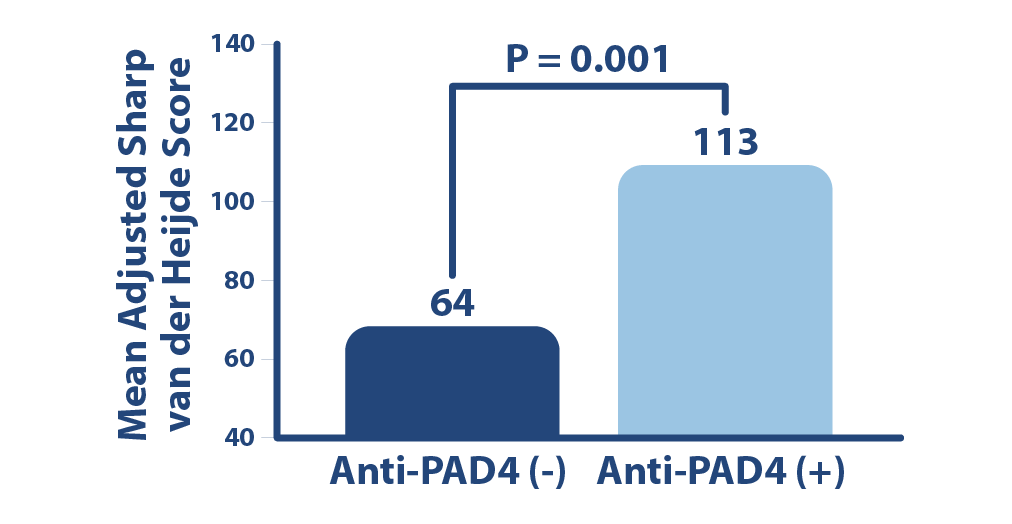
RA Severity Indicator
Anti-PAD4 is associated with heightened risk for severe joint damage. Anti-PAD4 autoantibody levels are independently associated with radiographic severity in RA.2
Anti-RA33 autoantibodies deliver greater diagnostic clarity for RA patients
Anti-RA33 autoantibodies (IgG, IgM, and IgA) target the RA33 protein complex, which plays an important role in gene expression and regulation.
Anti-RA33 autoantibodies are found in seronegative and seropositive patients with RA, providing a more complete assessment than conventional biomarkers alone.
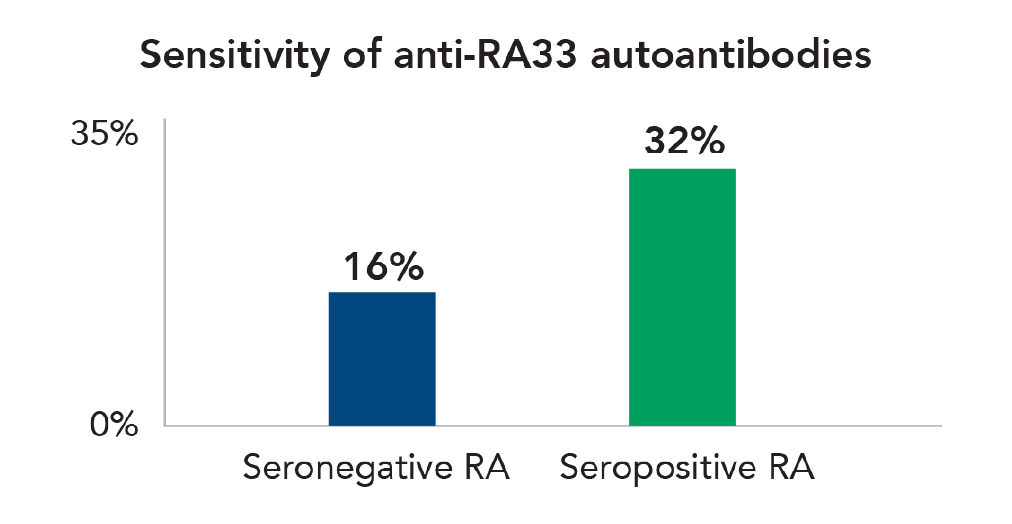
Sensitive
Anti-RA33 is found in 16% of seronegative and 32% of seropositive cases.1
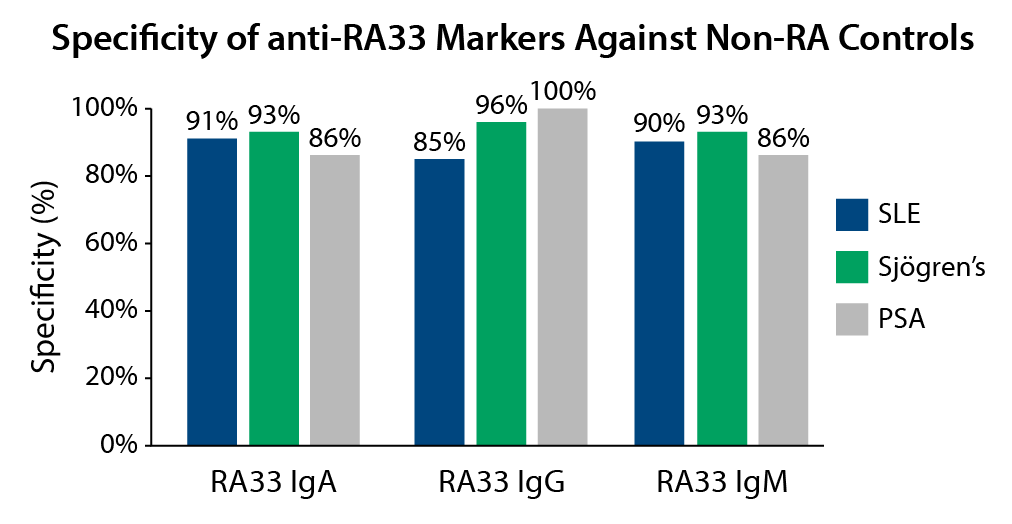
Specific
Anti-RA33 isotypes are ≥95% specific for RA against healthy individuals3 and ≥85% specific for RA against other autoimmune rheumatic diseases.3
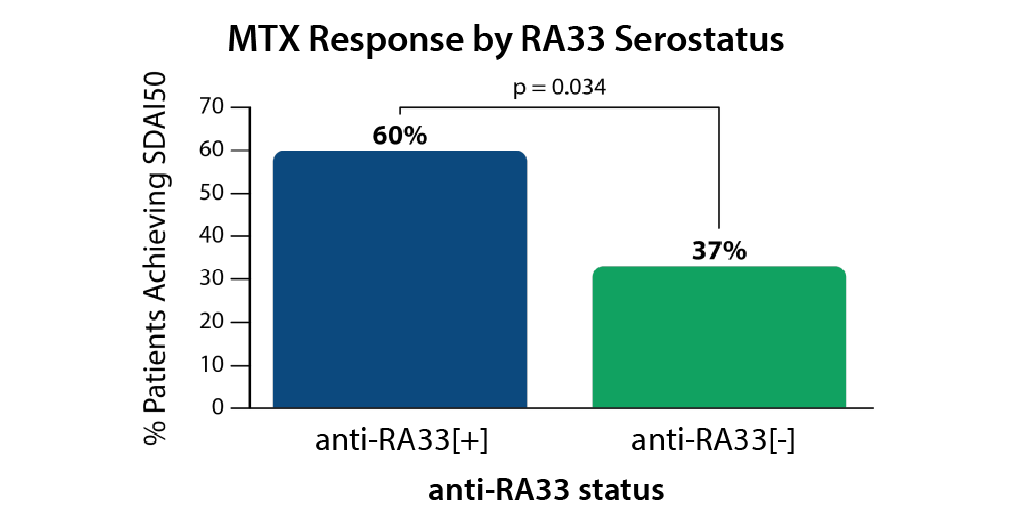
Collectively Valuable
Anti-RA33 positive patients show milder disease and a better treatment response, especially to methotrexate. 4
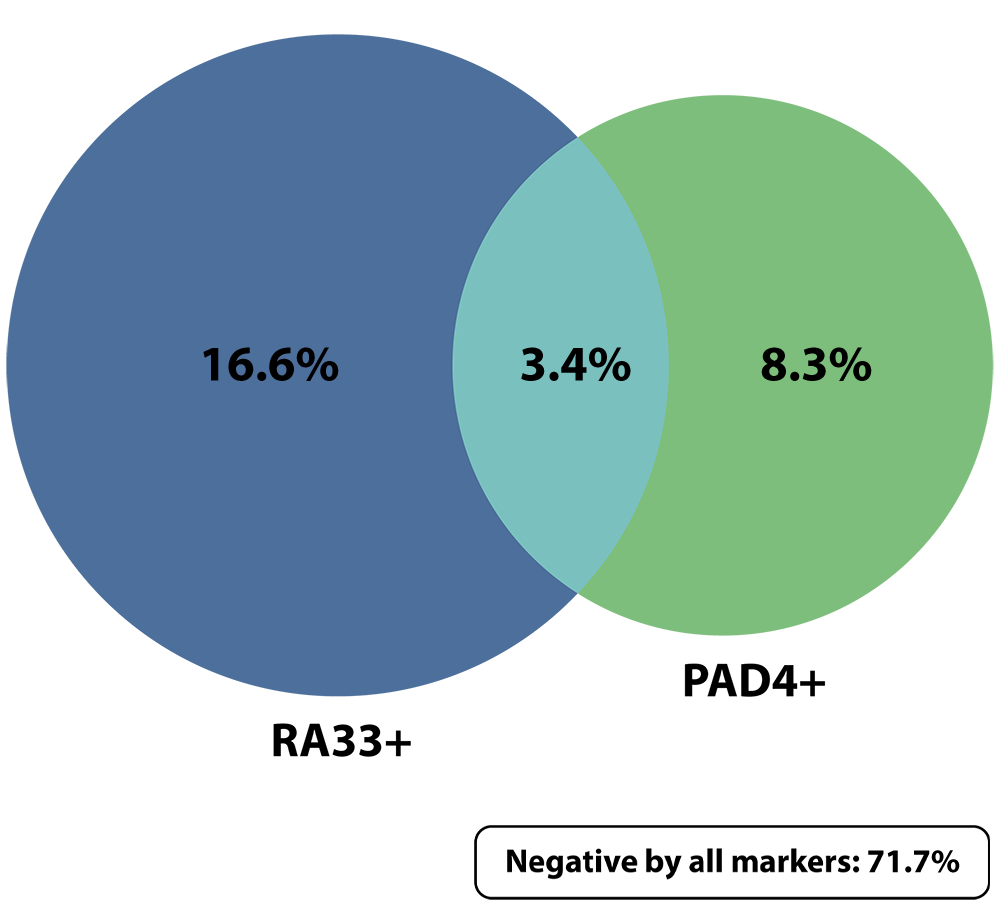
Anti-RA33 and anti-PAD4 are complementary autoantibodies that enhance the AVISE CTD panel to identify more seronegative RA patients
The RA profile combines novel biomarkers including anti-PAD4 and anti-RA33 with established anti-CCP and RF to help identity RA and substantiate a seronegative RA diagnosis.
Anti-RA33 and anti-PAD4 are complementary biomarkers with minimal overlap, which enhances their diagnostic capability when evaluated simultaneously.
Seropositive RA [N = 205]1
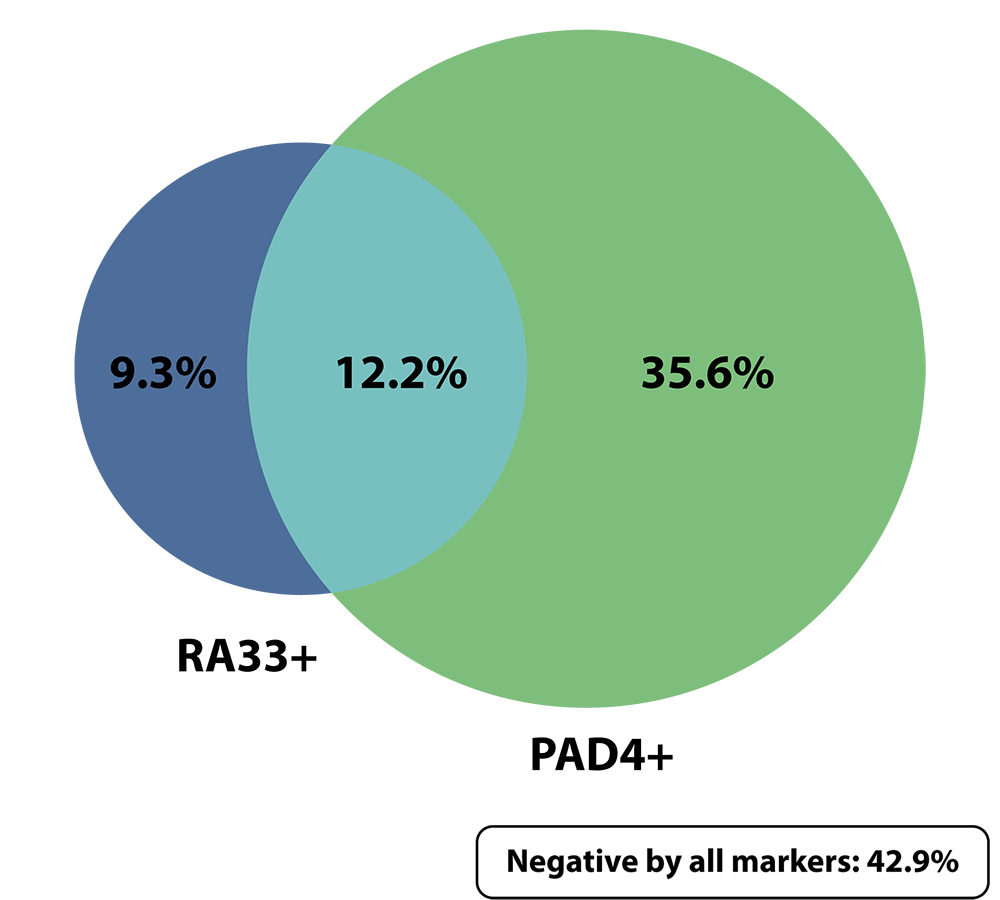
Seronegative RA [N = 145]1
Other Resources

ON-DEMAND WEBINAR Innovative Clinical Biomarkers for SLE and RA: Advancing Autoimmune Diagnostics
In this webinar, you gain insights into emergent biomarkers for systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). The speakers will share how novel T Cell biomarkers can offer more confident diagnoses of SLE, particularly when conventional biomarkers are insufficient.
ON-DEMAND WEBINAR Bridging the Gap in Seronegative Rheumatoid Arthritis: Unlocking the Diagnostic and Prognostic Potential of RA33 Antibodies
This webinar explores the emerging diagnostic and prognostic value of anti-RA33 autoantibodies (IgG, IgA and IgM) as novel biomarkers, capable of bridging the critical gap in seronegative rheumatoid arthritis (SN RA) clinical practice. Through a comprehensive review of existing diagnostic methods and their limitations, participants will understand why innovative biomarkers are urgently needed.
References:
- Data on file.
- Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008 July; 58 (7)1958–67. doi: 10.1002/art.23596.
- Concoff A, Warsi T, Taghavi S, et al. Anti-RA33 autoantibodies as biomarkers for seronegative rheumatoid arthritis in a U.S. cohort [abstract]. Arthritis Rheumatol. 2024; 76(suppl 9).
- Sieghart D, Platzer A, Studenic P, et al. Determination of autoantibody isotypes increases the sensitivity of serodiagnostics in rheumatoid arthritis. Front Immunol. 2018; 9:876.

