AVISE CTD RA33 Biomarkers
Getting to a rheumatoid arthritis (RA) diagnosis in seronegative patients can be challenging. To help address this, Exagen has identified new RA33 biomarkers. As part of AVISE CTD, these biomarkers aim to provide clinicians with a more comprehensive offering to help expedite their RA patients' diagnostic journey.
The diagnostic uncertainty of seronegative RA, often leads to delayed treatment, increasing the risk of joint damage, functional decline, and worsened disease outcomes. Exagen has addressed this issue by launching AVISE CTD's enhanced RA profile, which incorporates three new autoantibodies that are highly specific for RA, and can substantiate an RA diagnosis in the absence of anti-cyclic citrullinated peptide (anti-CCP) and rheumatoid factor (RF). Anti-RA33 autoantibodies (IgG, IgM and IgA) target the RA33 protein complex, which plays an important role in gene expression and regulation. Anti-RA33 autoantibodies are found in both seropositive and seronegative RA patients, providing a more complete assessment of individuals with this disease.
Anti-RA33 autoantibodies deliver greater diagnostic clarity for RA patients
Given the inherent challenges of seronegative RA, assessment of anti-RA33 antibodies is an essential component of comprehensive connective tissue disease evaluation. Anti-RA33 antibodies yield the ability to substantiate clinical suspicion of RA, facilitating earlier diagnosis and treatment.
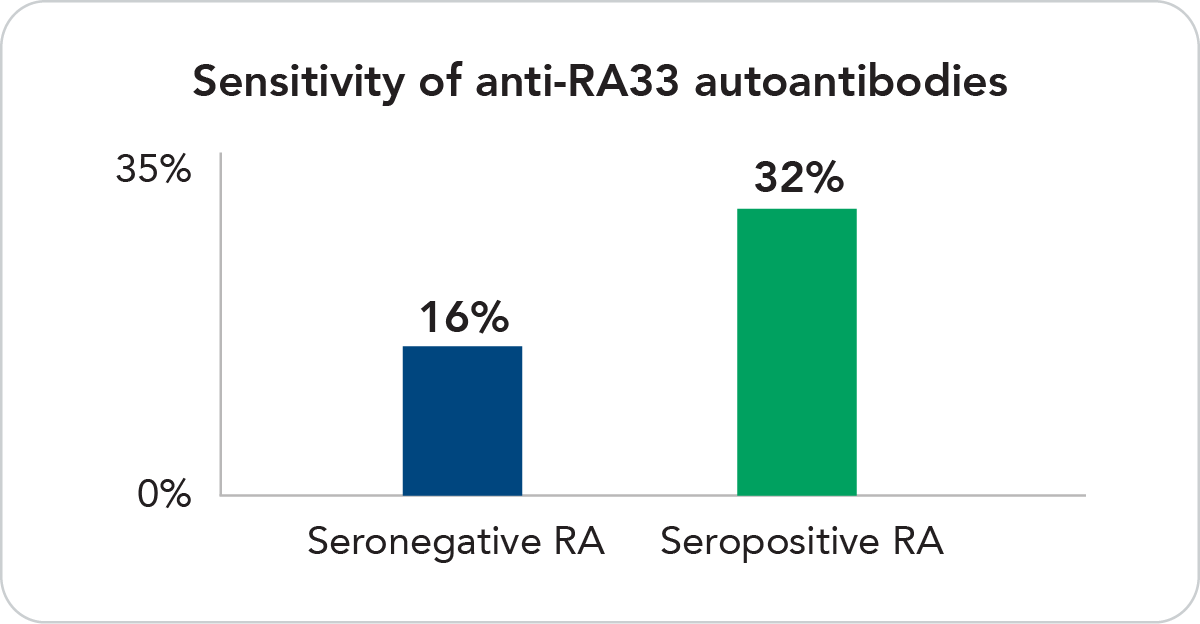
Uniquely Sensitive
Anti-RA33 IgG, IgM and IgA are found in 16% of the seronegative and 32% of the seropositive population.
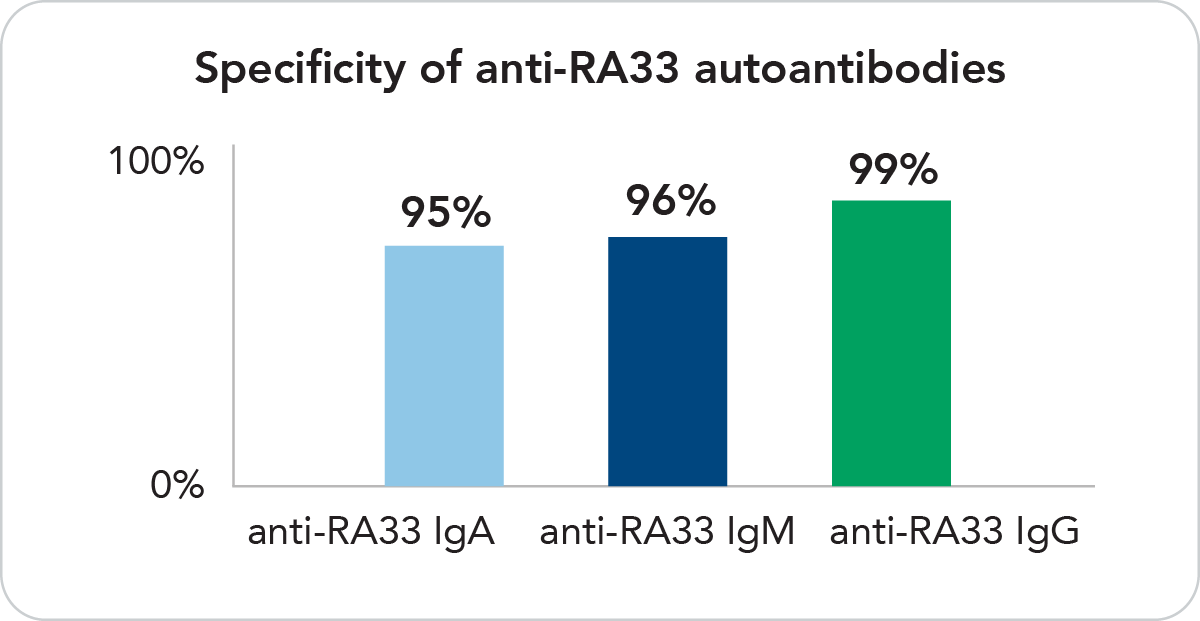
Highly Specific
Anti-RA33 IgG, IgM and IgA are 95-99% specific for RA against healthy individuals.
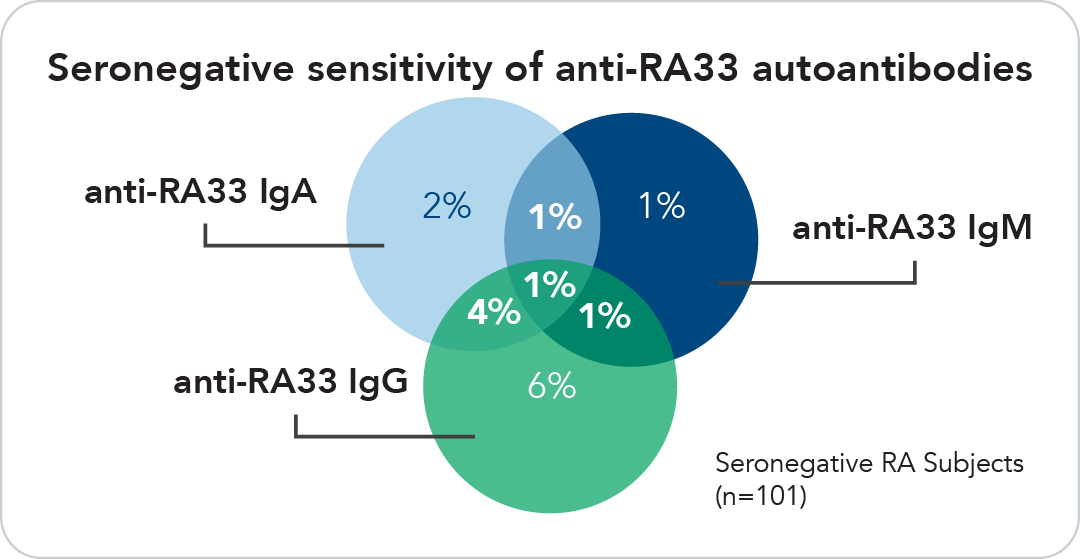
Collectively Valuable
The degree of overlap among RA patients for different anti-RA33 isotypes is minimal. The diagnostic utility of these autoantibodies is maximized when used together.
Other Resources

ON-DEMAND WEBINAR Innovative Clinical Biomarkers for SLE and RA: Advancing Autoimmune Diagnostics
In this webinar, you will gain insights into emergent biomarkers for systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). The speakers will share how novel T Cell biomarkers can offer more confident diagnoses of SLE, particularly when conventional biomarkers are insufficient.
ON-DEMAND WEBINAR Bridging the Gap in Seronegative Rheumatoid Arthritis: Unlocking the Diagnostic and Prognostic Potential of RA33 Antibodies
This webinar will explore the emerging diagnostic and prognostic value of anti-RA33 autoantibodies (IgG, IgA and IgM) as novel biomarkers, capable of bridging the critical gap in seronegative rheumatoid arthritis (SN RA) clinical practice. Through a comprehensive review of existing diagnostic methods and their limitations, participants will understand why innovative biomarkers are urgently needed.
References:
1. Sieghart D, Platzer A, Studenic P, et al. Determination of Autoantibody Isotypes Increases the Sensitivity of Serodiagnostics in Rheumatoid Arthritis. Front Immunol. 2018;9:876. Published 2018 Apr 24.
doi:10.3389/fimmu.2018.00876
2. Concoff A, et al. Anti-RA33 autoantibodies as biomarkers for seronegative rheumatoid arthritis in a U.S. cohort [abstract]. Arthritis Rheumatol. 2024; 76(suppl 9).
5. Data on File.

