Giving Patients the Answers They Need
Obtain Diagnostic Clarity with AVISE® CTD
Overlapping symptoms contribute to diagnostic uncertainty
Delays in diagnosis can lead to increased morbidity, higher mortality rates, and escalating healthcare costs. AVISE CTD addresses the challenge of overlapping symptoms by providing a comprehensive test to help you build the foundation for a diagnosis.

Just Two Tubes Provide Powerful Insights
With over 30 biomarkers carefully selected by rheumatologists for their diagnostic relevance, AVISE CTD helps improve diagnostic accuracy, reducing uncertainty and aiding in earlier diagnosis.
Here's how two tubes of blood can help you give your patients the answers they need:
CB-CAPs Biomarkers
EC4d • BC4d • TC4d
Proprietary Two-tier Algorithm
AVISE Lupus Index
Connective Tissue Disease Panel
RA • APS • MCTD • Sjögren's • Myositis • Systemic Sclerosis • Autoimmune Thyroid Disease • Lupus
Novel Biomarkers for Rheumatoid Arthritis
Approximately 30% of diagnosed RA and up to 50% of early RA cases are seronegative. The use of conventional and novel biomarkers in one assessment provides an opportunity to substantiate clinical suspicion of RA, aiding in earlier diagnoses and treatment.
AVISE CTD’s enhanced RA profile incorporates conventional biomarkers (anti-CCP and RF) and new biomarkers (anti-RA33 and anti-PAD4) that are specific and sensitive to RA even in patients who test negative for anti-CCP and RF.
Anti-CarP autoantibody testing can be added to AVISE CTD to further enhance diagnostic profiles in seronegative and early RA cases.
AVISE® CTD Includes Novel Biomarkers and the Lupus Index Score
New T Cell Biomarkers for Systemic Lupus Erythematosus
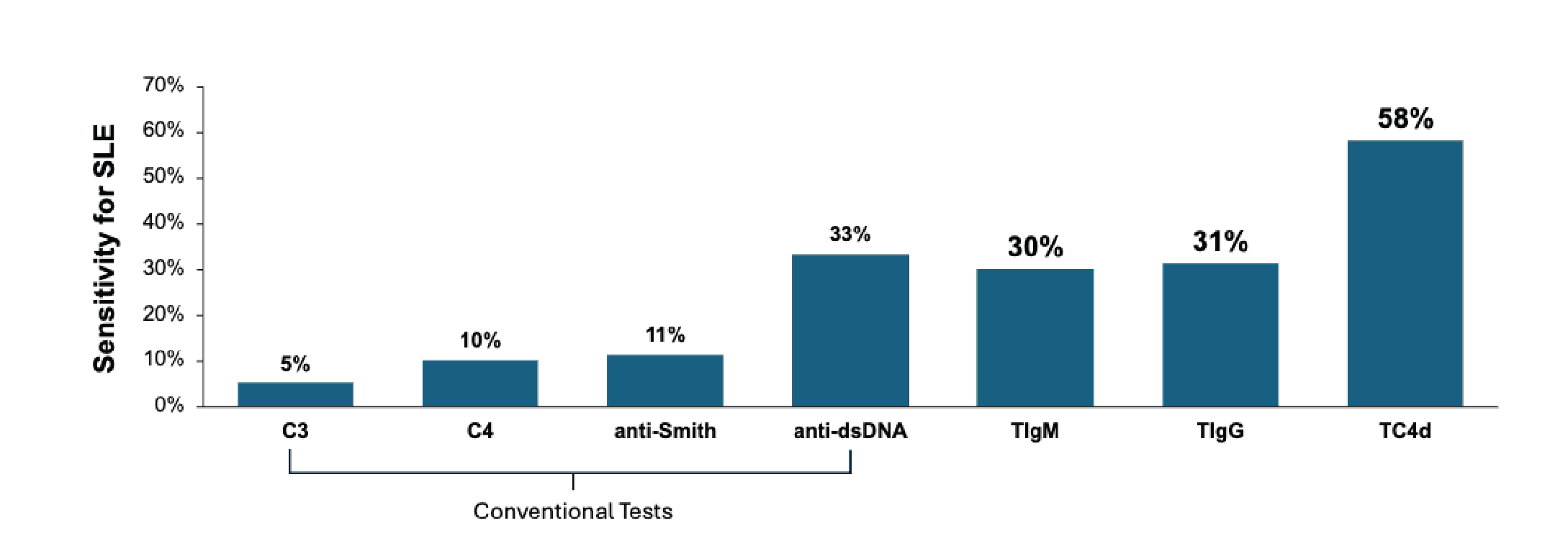
Conventional SLE biomarkers such as anti-dsDNA, anti-Smith, and complement C3/C4 have limited discriminatory power characterized by poor sensitivity in incipient cases.
Newly available T Cell bound complement and autoantibodies have superior diagnostic accuracy when conventional biomarkers are uninformative, facilitating earlier diagnoses and treatment decisions.
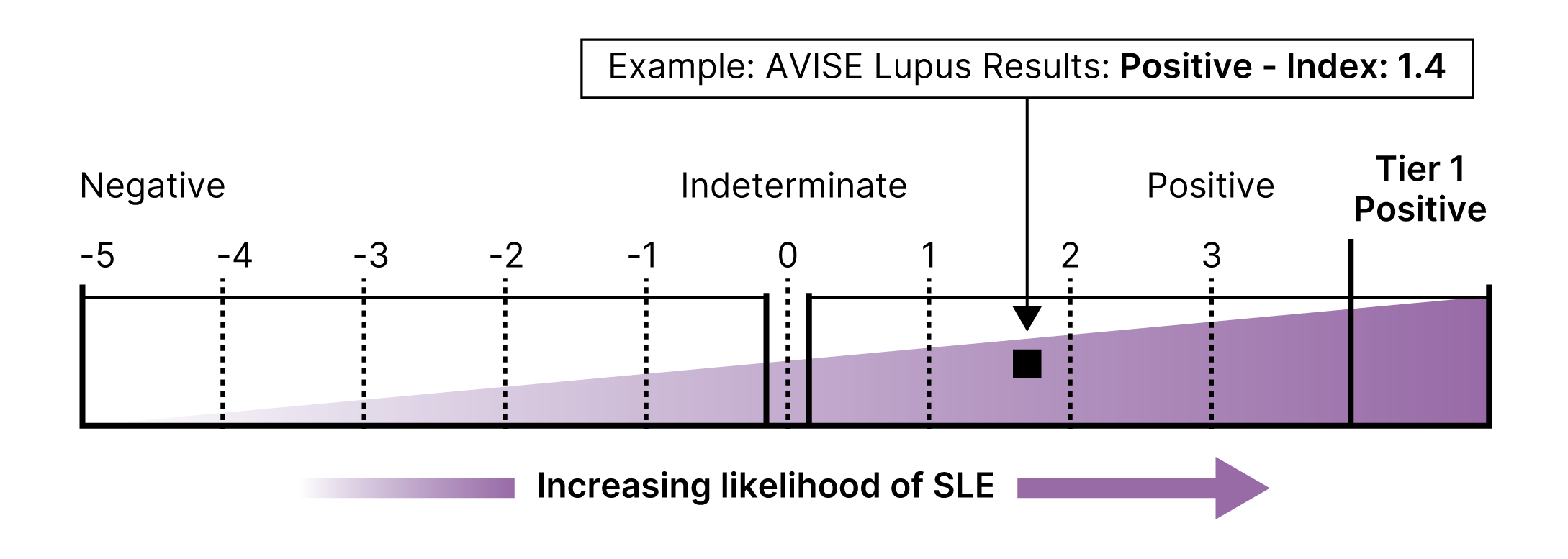
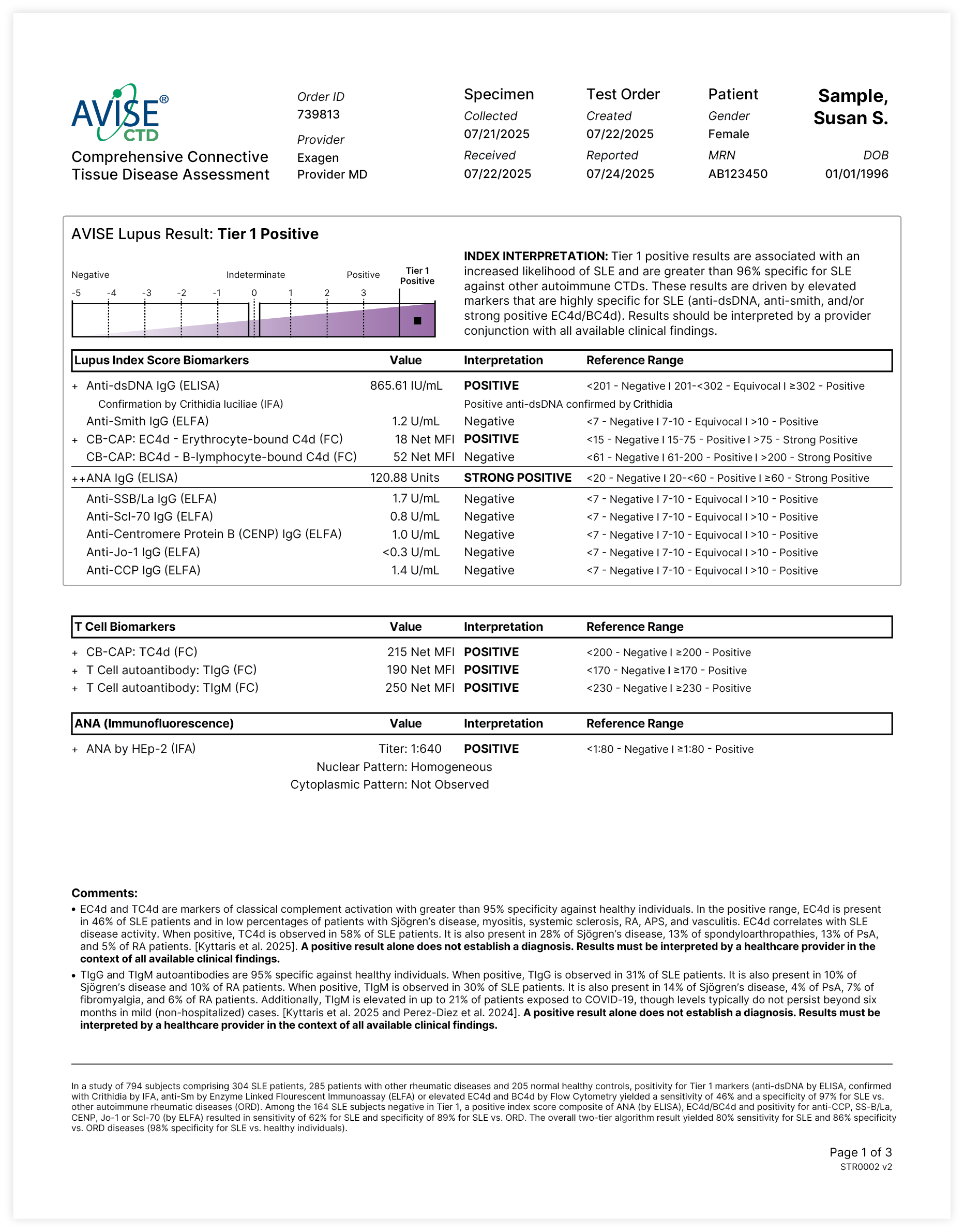
Proprietary Lupus Algorithm
AVISE CTD employs an advanced two-tier algorithm and proprietary Index Value to convert isolated data points to an index score, which reports the serologic likelihood of the presence of SLE.
This multi-marker algorithmic assay integrates CB-CAPs and autoantibody levels to provide a diagnostic result that is both sensitive and specific for an SLE diagnosis.
Build Confidence With Reliable Results
Exagen has extensive peer-reviewed literature supporting the clinical utility of its proprietary technology, demonstrating actionable results that support your diagnostic and treatment decisions.